| |
February
2014
- Volume 8, Issue 1
Fatigue in
Early Stage among Jordanian Patients with Cancer Receiving
Chemotherapy

|
 ((2) ((2)
|
Kholoud Abu Obead (1)
Sameer Yaser (2)
Maysaa Khattab (3)
Faisal Albadainah (4)
Laila Saqer (5)
Nehaya Al-dosouqi (6)
(1) Kholoud Abu Obead, RN, MSN, CNS;
(2) Sameer Yaser, MD. Consultant Medical Oncology;
(3) Maysaa Khattab , RN, MSN;
(4) Faisal Al-badaynah, MD. Consultant Medical Oncology;
(5) Laila Saqer RN, MSN, CNS;
(6) Nehaya Al-dosouqi RN.
Correspondence:
Kholoud Abu Obead RN,
MSN
Fulltime lecturer
Jordan University of Science And Technology
Faculty of Nursing
Email: kabuobeid@yahoo.com
|
 |
|
Abstract
The purposes of this study were to (1) examine the impact
of Chemotherapy on fatigue in Jordanian cancer patients,
and (2) to chemotherapy related fatigue (CRF) with selected
demographic variables such as age, sex, marital status,
income, level of education, type of cancer, stage of
disease , type of chemotherapy, body mass index, smoking
and hemoglobin level. One group quasi-experimental co-relational
design was used with 43 patients who had been diagnosed
with cancer and required Chemotherapy treatment. Fatigue
was measured using Piper Fatigue Scale (PFS). Data was
collected over a period of six months and analyzed using
descriptive statistics, paired-sample t-test, and Pearson
Product Moment Correlation. Statistically significant
differences were found between total fatigue scores
as well as on behavioral, affective, sensory, and cognitive
dimensions of PFS, before starting chemotherapy treatment
and after 4 weeks from receiving the first dose of chemotherapy
treatment.
Key words: Jordan, cancer patients, fatigue,
chemotherapy
|
Introduction
Fatigue is one of the most prevalent symptoms of patients
with cancer (1). It occurs across all ages,
genders, cancer diagnoses, stages of disease, and treatment
regimens (2). Cancer Related Fatigue (CRF) is different from
everyday tiredness, which can be reversed by rest or sleep.
It is characterized by an overall lack of energy, cognitive
impairment, somnolence, mood disturbance, or muscle weakness
(The National Comprehensive Cancer Network (NCCN), 2013).
It is a multidimensional phenomenon, which evolves over time,
compromising physical energy, mental capacity and the psychological
condition of the patient with cancer (3).
Studies showed that 82-96% of patients receiving chemotherapy
or radiotherapy (4, 5) suffer from fatigue during their treatment.
And in the same magnitude; patients with metastatic disease
suffered from fatigue (6). CRF is under reported and is under-evaluated
by health care givers (7) despite the presence of growing
evidence on the impact of CRF on quality of life (QoL) (8).
Cancer Related Fatigue can be caused or potentially predisposed
by various factors. A multidimensional model which includes
situational, biological, physical symptoms and psychological
symptoms, has been explored for CRF, beside that situational
dimensions; inpatient status, analgesic use and stage of cancer
were also correlated significantly with fatigue level (9).
For a biological dimension hemoglobin level was an independent
predictive factor for CRF (P = 0.02) (9). The impact of anemia
on CRF may be different depending on onset time, patient age,
and co-morbidity (10).
Despite the high prevalence of fatigue and potential negative
effect on patients' activities and emotional well-being, research
in fatigue is still underdeveloped and there are no studies
reporting on CRF among Jordanian population. So, this study
is an attempt to explore fatigue among Jordanian cancer patients
who are being treated with chemotherapy in Jordan. In addition,
it is anticipated that this study will have the potential
to motivate staff to take fatigue into consideration while
providing care for oncology patients.
Methods
Design
One group quasi-experimental correlational design was used
to examine the impact of chemotherapy treatment on Jordanian
cancer patients' fatigue, and to examine the relationship
between selected demographic variables and fatigue.
Sample
A consecutive sampling procedure was used to recruit potential
participants for this study. The inclusion criteria are as
follows: (a) 18-65 years old, (b) has no history of psychiatric
or mental problem, (c) has chemotherapy for the first time,
(d) is treated with chemotherapy only, (e) has Hemoglobin
(Hb) level above 12 g /dl at the beginning of the study, (g)
has no history of cardiac, respiratory or medical illnesses,
(h) is able to give verbal consent to participate in this
study, and (i) diagnosed with solid and metastatic disease.
The sample size was determined by Cohen (1988) formula. Cohen
identified three levels for the effect of the sample size
when using Paired Sample T test: small 0.2, medium 0.5, and
large 0.8. Based on this classification and literature review,
the medium effect size for comparison between two means was
anticipated for this study. Testing one tailed hypothesis
at significant level of alpha 0.05, the sample size was determined
to be 43 participants. Therefore, the convenience sample of
43 participants who were treated with chemotherapy at KHCC,
and met the inclusion criteria, agreed to participate, and
were able to complete the study measurements participated
in this study. The researcher interviewed each participant
using the designated questionnaires PFS and DDS of the study,
twice, immediately before receiving first cycle of chemotherapy
and after 4 weeks from receiving first dose of chemotherapy
treatment.
Setting
The King Hussein Cancer Center (KHCC) is a medical center
located in Amman City, the capital of Jordan. It treats both
adult and pediatric patients. KHCC treats over 3500 new cancer
patients each year from Jordan and the region. KHCC has established
programs that focus on all stages of comprehensive cancer
care: from prevention and early detection, through diagnosis
and treatment, to palliative care.
Instrumentation
The following instruments were used to collect data from all
participants in this study:
1. Demographic Data Sheet
The Demographic Data Sheet (DDS) was developed by the researcher
to elicit background information about the patients. The DDS
includes questions related to age, marital status, gender,
level of education, monthly income, occupation, religion,
type of cancer, stage of disease, complications of cancer,
type of chemotherapy y, dose of chemotherapy, chemotherapy
side effects, body mass index, hemoglobin level at the beginning
of treatment, hemoglobin level after 4 weeks from receiving
first dose of chemotherapy treatment
2. Piper Fatigue Scale (PFS)
The Piper Fatigue Scale (PFS) is a multidimensional tool designed
to measure the level of fatigue subjectively, and has been
widely used in research. It has the potential to differentiate
three levels of fatigue; mild, moderate and severe (11). Piper
Fatigue Scale (PFS) is congruent with the conceptual framework
of this study, which acknowledges fatigue as a subjective
phenomenon.
After gaining permission from the original author, the instrument
was translated to Arabic to minimize barriers of assessment
with Arabic participants. The translated version of the instrument
was back translated to ensure content and semantic validity.
Content validity was assessed by a panel of experts in nursing
who reviewed the items for clarity, relevance, comprehensiveness,
understandability, and ease of administration. The panel of
experts recommended no modifications.
Before embarking on the full study, a pilot test of the Arabic
version was conducted with 10 participants within the target
population to ensure that the tool is readable and can be
understood by those who will use it. The pilot study indicated
that Arabic version of PFS was in general readable, and easily
understood. Participants did not request any additional information
to be included in the questions. Structured interview for
each participant required from 10 to 15 minutes. Reliability
coefficient alpha was calculated for total PFS scores and
subscales scores. The results showed that the Arabic version
of PFS is a reliable instrument, with internal consistency
of the entire Arabic version of PFS (alpha =0.947), and for
the four subscales: behavioral, affective, sensory, and cognitive
dimension (alpha = 0.915, 0.807, 0.952, and 0.864) respectively.
Ethical consideration
The study protocol was approved by the Institutional Review
Board at King Hussein Cancer Centre administration, to conduct
the study. Daily visits were made to the setting to check
for participants who met the inclusion criteria. Once a participant
was identified, verbal consent was obtained after providing
adequate information about the significance and purposes of
the study. Participants were assured that participation was
voluntary, and participants were told to feel free to withdraw
at any time. Participants were assured that their responses
would be treated confidentially and information that might
reveal their identity would not be recorded and only aggregated
data would be communicated.
Results
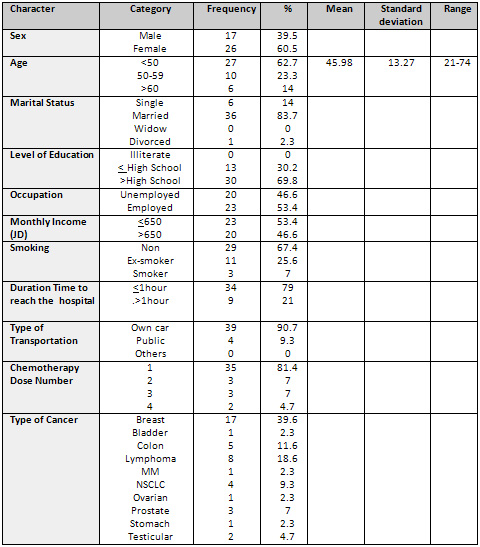
Participants' Characteristics
All participants were treated with different types of chemotherapy
at KHCC. The age of participants ranged from 21-73 years (M=
45.98, SD= 13.27). Most participants were female (n=26), married
(n= 36), had high school diploma (n=30), and were employed
(n=23); 23 participants had a monthly income less than 650
JD, about 93% were non-smokers, diagnosed with breast cancer
(n=17), obesity was present in about 64.4% of participants,
most of them were treated with Anthracyclin based regimen.
(See Table 1 for sociodemographic characteristics of the sample).
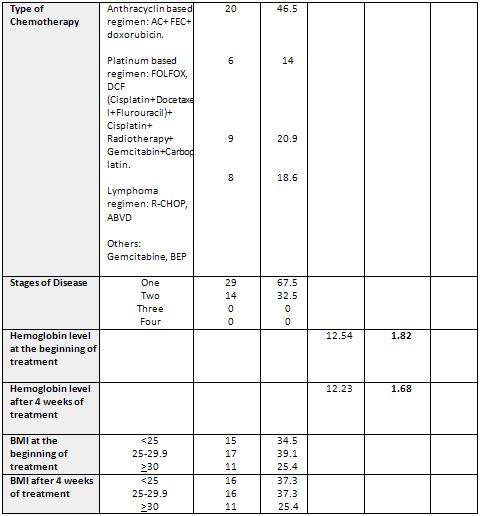
Table 1: Sociodemographic
Characteristics of the Sample
Baseline Measurements (pre-treatment)
Piper Fatigue Scale (PFS) Scores
The total PFS scores of participants ranged from 0.75
to 6.2(M=2.96, SD=1.45). Almost all participants scored low
on all subscales of PFS prior to receiving first dose of chemotherapy
treatment; the behavioral subscale scores ranged from 0.00
to 4.83 (M=1.27, SD= 1.1), affective subscale scores ranged
from 1.00 to 6.6 (M=2.86, SD=1.57), sensory subscale scores
ranged from 1.00 to 7.8 (M=3.8, SD=1.63), and cognitive subscale
scores ranged from 1.00to 8.2 (M= 3.9, SD=1.97), (see Table
2 for means and standard deviations of the scores on all subscales
of PFS prior to receiving first dose of chemotherapy treatment).
Table 2: Means and Standard Deviations
of the Scores on all Subscales of PFS prior to Receiving First
Dose of Chemotherapy treatment
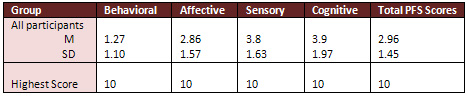
(N=43)
Post Treatment Measurements
The total participants' scores on
PFS after 4 weeks from receiving first dose of chemotherapy
treatment ranged from 1.83-7.08(M=5.26, SD=1.01). Almost all
participants scored high on all subscales of the PFS after
4 weeks from receiving first dose of chemotherapy treatment
with behavioural subscale that ranged from 0.17 to 6.83 (M=3.51,
SD=1.46), affective subscale scores ranged from 2.2 to7.8
(M=5.05, SD=1.27), sensory subscale scores ranged from 2.4
to 8.8 (M=6.19, SD=1.36), and cognitive subscale scores ranged
from 1.33to 8.5 (M= 6.31, SD=1.33), (see Table 3 for means
and standard deviations of the scores on all subscales of
PFS after 4 weeks from receiving first dose of chemotherapy
treatment).
Table 3: Means and Standard Deviations
of the Scores on all Subscales of PFS after 4 weeks from receiving
first dose of chemotherapy treatment
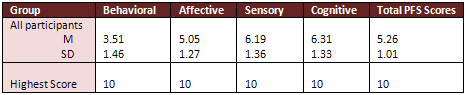
(N=43)
Research Question 1 (Fatigue Score).
To answer the first research question "Do patients who
receive chemotherapy as a primary treatment for their cancer
have statistically higher scores of fatigue as measured by
PFSs after 4 weeks from the first dose compared to their scores
at the beginning of their treatments? " A paired sample
t-test was used for total scores, and each subscale of PFS.
Paired sample t-test revealed significant differences between
respondents' total mean scores of fatigue pre and post 4 weeks
chemotherapy treatment as measured by total PFS questionnaire
(t= -2.31, df=42, P<0.05). In addition, significant differences
were found between pre and after 4 weeks from receiving the
first dose of chemotherapy treatment scores for behavioral,
affective, sensory, and cognitive dimensions subscales (t=
-2.24, -2.19, -2.4, -2.4, df =42, P<0.05) respectively,
(see Table 4 for the results of paired-sample t-test for fatigue
scores as measured by PFS).
Table 4
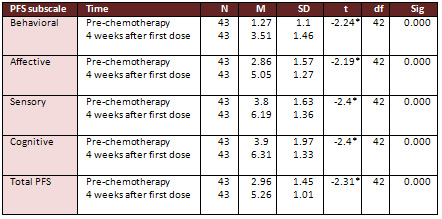
*P <0.05
Research Question 2
"Is there a relationship between fatigue scores (PFS)
and selected demographic variables such as age, sex, marital
status, Income, level of education, type of cancer, stage
of disease, type of chemotherapy, dose of chemotherapy, body
mass index, smoking and hemoglobin level among Jordanian patients
who receive chemotherapy as a primary treatment for their
cancer?". To find the relationship between fatigue score
and sociodemographic variables Pearson Product Moment Correlation
and Biserial Correlation Coefficient were used.
Pearson Product Moment Correlation Coefficient was used to
find the correlation between fatigue scores as measured by
PFS and selected sociodemographic variables on a continuous
level. Pearson Product Moment Correlation showed a significant
negative relationship between fatigue scores as measured by
PFS and hemoglobin level (r= -0.04, P<0.01). (See Table
5 for the results of Pearson Product Moment Correlation Coefficient
between fatigue Scores as measured by PFS and sociodemographic
variables on a continuous level).
Biserial Correlation Coefficient was used to find the correlation
between fatigue scores as measured by PFS and selected sociodemographic
variables on nominal and dichotomus levels. Biserial Correlation
Coefficient showed a significant negative relationship between
fatigue scores measured by PFS and sex (r= -0.026, P<0.01).
Also, Biserial Correlation Coefficient showed a positive relationship
between fatigue scores measured by PFS and type of chemotherapy
especially patients treated with Anthracyclin based regimen
( r= 0.0398, P<0.05). (See Table 6 for the results of Biserial
Correlation Coefficient between fatigue scores as measured
by PFS and sociodemographic variables on nominal and dichotomus
levels).
Table 5: Results of Pearson Product
Correlation Coefficient between Fatigue Scores as measured
by PFS and Sociodemographic Variables on a Continuous Level

** Correlation is significant at 0.01 level.
Table 6: Results of Biserial Correlation Coefficient between
Fatigue Scores as measured by PFS and Sociodemographic Variables
on Nominal and Dichotomus Levels
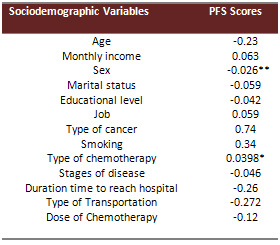
*Correlation is significant
at 0.05 levels.
** Correlation is significant at 0.01 level.
Discussion
Regarding question 1
The findings of this study showed
that the patients who received chemotherapy as a primary treatment
for their cancer have statistically higher scores of fatigue
as measured by PFS's after four weeks from the first dose
compared to their scores at the beginning of their treatments;
and thus demonstrated that fatigue is more related to treatment
of cancer than to the cancer and may persist after therapy
(12). The reason for increased fatigue scores after 4 weeks
from the first dose may be explained based on the fact that
the etiology of fatigue in cancer patients is complex, and
multidimensional (13). Previous studies (14; 15) found that
fatigue precedes, accompanies, and follows most tumours and
its treatment. Chemotherapy and radiotherapy cause cellular
death (14). As a consequence several chemicals are released
into circulation. Such chemicals may increase basal metabolic
rate, which may affect energy level (15).
Many cancer patients feel fatigued for several months or even
years after their treatment with chemotherapy (16). The previous
studies found that fatigue is the most common side effect
of cancer treatment including chemotherapy. The mechanism
of how chemotherapy causes fatigue is unknown (17) but some
studies explained that fatigue related to chemotherapy may
be caused by the need for extra energy for the process of
the healing and repairing body tissues that are damaged by
treatment in addition to the building up of toxic substances
that are left in the body after using of cancer treatment
for killing malignant cells(12).
Research question 2
Of the socio-demographic variables, sex correlates negatively
with fatigue. This finding is consistent with many previous
studies that showed; women who received chemotherapy reported
higher fatigue severity scores than men (18). In this study
it could be explained by the differences in the ratio of female
participants to male; where most of the study sample are females.
Anemia can occur as a result of the cancer or the cancer treatment
(12). Anemia was found to be a common cause of fatigue (12).
In this study; the patients with low hemoglobin level perceived
a higher level of fatigue than those with high hemoglobin
level. This result confirmed the results of previous studies
(19). This could be explained by when patients become anemic
there is a decrease in the number of circulating red blood
cells, the oxygen carrying capacity of the blood is diminished
and thus make the patient's heart and lungs work harder and
make patients feel tired and weak due to the inadequate supply
of oxygen to muscles and other organs (12, 19).
There was a relationship between fatigue and type of chemotherapy
(Anthracyclin based regimen). Previous studies demonstrated
that fatigue is more related to treatment than to cancer and
it may be last after therapy (20). Anthracyclin containing
chemotherapy is well known to cause dose dependent progressive
cardiac damage, heart failure and cardio toxicities which
in turn play an important role in decreased oxygenated blood
supply to all body tissues and finalized with fatigue (21).
Treatment with the Anthracycline can result in the production
of toxic substances as within the cancer cells (20). The more
and longer accumulative dose of Anthrocyclin, the more destructive
effect on the body (21). There was no available previous studies
that assessed fatigue associated with low dose of Anthracyclin.
No relationship was found in this study between BMI and fatigue
that could be related to the short duration (four weeks )
between pre and post treatment with chemotherapy which is
not enough to detect changes in BMI and so changes in perceiving
level of fatigue.
Limitations
1. The use of convenience sample and the small sample
size were a major limitation since only 43 participants were
able to complete this study. So the generalizability of the
findings of this study is limited. The inferential statistics
performed on these data must, therefore, be interpreted with
extreme caution, and no conclusions can be drawn with certainty.
Therefore, these limitations were threatening the generalizability
of the findings.
2. Validity and reliability of PFS need to be tested
in further study.
Conclusions
Despite the limitations of this study, the current and previous
research findings, as well as the well-established facts about
cancer and chemotherapy, the following conclusions can be
drawn:
1. Cancer patients receiving chemotherapy are at risk
for considerable treatment related fatigue. Therefore, health
care providers should incorporate fatigue in routine assessment
of patients who are being treated for cancer or being followed
after completing treatment.
2. Fatigue is influenced by hemoglobin level and gender.
Therefore, health care providers have an obligation to take
these variables into account when caring for cancer patients.
Recommendations
1. Replicating this study with large samples is necessary
before making any generalization of the results.
2. Healthcare providers should incorporate fatigue
in routine assessments of patients who are being treated for
cancer or being followed after completing treatment.
3. Help healthcare providers to consider how people
understand, interpret feelings, and sensations associated
with fatigue.
4. Teach patients, parents, and health care professionals
about the symptoms and impact of fatigue and the treatable
nature of fatigue.
5. Develop new instruments to assess Jordanian cancer
patients' fatigue from their cultural perspectives.
6. Assess patient' responses to fatigue taking into
consideration verbal and non-verbal responses that vary from
one patient to another.
7. Further research is needed to compare levels of
fatigue related to chemotherapy and other cancer therapy.
Implications
The following are implications for nursing and medical research,
education, practice, and administration based on the results
of this study:
1. Health Care Providers should assess fatigue for
all cancer patients periodically during their disease and
treatment.
2. Course design individualized nursing and medical
care plan for their patients taking into consideration fatigue.
3. Health care educators are advised to incorporate
fatigue issues in nursing and medical educational programs.
4. Results of this study indicated the need for further
studies to explore the effectiveness of nursing and medical
strategies used to cope with fatigue among patients in general,
and cancer patients in particular.
5. Further studies are needed to assess knowledge,
and attitude toward fatigue among nurses and doctors.
6. Hospital administrators must encourage workshops
for nurses and other health team members who are responsible
for patients' education in strategies used to cope with fatigue.
7. Hospital administrators are encouraged to develop
teaching materials like pamphlets, booklets, and brochures
about chemotherapy to reduce fatigue in cancer patients and
increase their knowledge about it.
8. Establish staff development for nurses in the oncology
centers to assist cancer patients to develop fatigue reduction
and management programs.
9. Health care administrator should develop an assessment
tool to predict patients who are at increased risk for experiencing
high fatigue levels during and after cancer treatment.
References
1. Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ,
Itri LM, et al. Impact of cancer-related fatigue on the lives
of patients: New findings from the Fatigue Coalition. Oncologist.
2000;5:353-60.[PubMed].
2. Winningham ML. Strategies for managing cancer-related fatigue
syndrome: A rehabilitation approach. Cancer. 2001;92:988-97.[PubMed].
3. Fatigue in cancer patients receiving chemotherapy: an analysis
of published studies. Annals of Oncology 2004 ; 15 (5):712-720.
4. Nerenz DR, Leventhal H, Love RR. Factors contributing to
emotional distress during chemotherapy. Cancer 1982; 50: 1020-1027.
5. Winningham M. Fatigue and the cancer experience: The state
of knowledge. Oncology Nursing Forum 1998; 21:1: 23-36.
6. Kazi S Manir, Kallol Bhadra, Gaurav Kumar, Amitabha Manna,
Niladri B Patra, and Shyamal K Sarkar. Fatigue in Breast Cancer
Patients on Adjuvant Treatment: Course and Prevalence. Indian
J Palliat Care. 2012 May-Aug; 18(2): 109-116.).
7. Portenoy RK, Itri LM. Cancer-related fatigue: guidelines
for evaluation and management. Oncologist1999; 4: 1-10.
8. Doranne L, Paul H. Kloeg, Elsken Van der Wall, Chad M Gund
, Manon Komen and Neil K Aaronson. Cancer Related Fatigue
: Clinical Practice Versus Practice Guide Line under reported
presence of growing evidence on the impact of quality of life.
Support care Cancer: April 2011; 19(4): 531-538.
9. Hwang SS, Chang VT, Rue M, Kasimis B. Multidimensional
independent predictors of cancer-related fatigue. J Pain Symptom
Manage. 2003 Jul;26(1):604-14.
10. Berndt E, Kallich, J, McDermott A, Xu X, Lee H, Glaspy
J (2005). Reductions in Anemia and Cancer-related fatigue
are Associated with Improvements in Productivity in Cancer
Patients Receiving Chemotherapy. Pharmacoeconomics; 23(5):505-514.
11. Piper F. Piper Fatigue Scale: Psychometric Evaluation
in Women with Breast Cancer. PIPER, 1998; 25: 4: 677-684.
12. Ernest H. R, Barbara F., Marilyn D, Kathleen D, Michael.
G, Pat. Kr, Rose . A Kurshner, Francine M, . Fatigue Reduction
and Management For The Primary Side-Effects Of Cancer Therapy.
Cancer supportive survivorship care.www.cancersupportivecare.com/fatigue.html.
13. Bottomley A, Flechtner H. Fatigue and QOL: Lessons from
the real world. [On Line] [accessed 2003 August]. Available
from URL http//: www. ncbi. Nlm.nih.gov/ fcgi.
14. Aistar J. Fatigue in Cancer patients: a conceptual approach
to clinical problem. Oncol Nurs Forum 1987; 14: 25-30.
15. Ferrel B, Dow K, Leigh L, Gulasekarm P. Quality of life
in long term cancer survivors. Oncology Nursing Forum 1995;
22: 915-922.
16. Rose Ann Kurshner, Francine Manuel. Cancer supportive
and survivorship care. (2008).www.cancersupportive care.com.
17. Fatigue (PDQ).National Cancer Institute (NCI,s). www.cancer.gov/cancer.
18. Christine Miaskowski. Gender Differences in Pain, Fatigue,
and Depression in Patients with Cancer. Oxford Journals, JNCI
Monographs. 2004;(32):139-43.
19. Health wise Incorporation. Cancer: How to Deal With Fatigue
and Anemia - Network Health. www.network-health.org.
20. T. M. Suter & B. Meier. Detection of Anthrocyclin
induced cardiotoxicity: is there light at the end of the tunnel?
Oxford Journals Medicine Annals of Oncology,2002, Volume 13,
Issue 5.pp.647-649.
21. Jessica M. Scott, John R. Mackey, Mark J. Haykowsky, Pamela
S. Douglas, Lee W. Jones. Modulation of Anthrocyclin Induced
Cardiotoxicity by Aerobic Exercise in Breast Cancer; Current
Evidence and Underlying Mechanisms. Circulation. 2011; 124:
642-65.
|
 |




