| |
May
2016 - Volume 10, Issue 2
Methylnaltrexone or laxatives for the Management of Opioid-induced
Constipation among Palliative Patients on Opioid Therapy:
Evidence-based Review

|
 ( (
|
Mohammad
G. H. Tuffaha (1)
Nijmeh Al-Atiyyat (2)
(1)
Mohammad G. H. Tuffaha, R.N, BSc, MSN.
Hashemite University, School of Nursing, Zarqa, Jordan
Al-Bashir Hospital, Department of Hematology, Amman,
Jordan
(2) Dr. Nijmeh Al-Atiyyat, BSc, MSN, PHD.
Hashemite University, School of Nursing, Zarqa, Jordan
Correspondence:
Mohammad G.
H. Tuffaha, R.N, BSc, MSN.
Hashemite University, School of Nursing, Zarqa, Jordan
Al-Bashir Hospital, Department of Hematology, Amman,
Jordan
Email: mohammad_toffaha@yahoo.com
|
 |
|
Abstract
Constipation is a common symptom in advanced cancer
patients. Studies have demonstrated that 40 to 80% of
patients on a palliative care service have constipation.
This proportion increases to > 90% when patients
are treated with opioids. Opioids are very effective
analgesics, frequently prescribed in cancer pain. Despite
proven analgesic efficacy the use of opioids is commonly
associated with frequently dose-limiting constipation
that seriously impacts on patients' quality of life.
Almost all patients on opioids report constipation as
the major side-effect. The aim of this article is to
determine the effectiveness of methylnaltrexone and
laxatives in the management of opioid-induced constipation
(OIC) among cancer patients in palliative care setting,
with focus on randomized clinical trials. A comprehensive
and extensive online database search of Science Direct
Database, PubMed, Springer Online Database, and HINARI/WHO
Database was conducted; also reference lists of related
studies were searched. Six studies fulfilling the inclusion
criteria from 1991 to 2009 were selected and formed
the basis for this paper. In three studies the laxatives
lactulose, senna, co danthramer, misrakasneham, and
magnesium hydroxide with liquid paraffin were evaluated,
and thirdly methylnaltrexone
. In studies comparing the different laxatives evidence
was inconclusive. Evidence on subcutaneous methylnaltrexone
was clearer; evidence on laxatives for management of
constipation remains limited due to insufficient RCTs.
Ultimately it can be suggested from the data presented
here that subcutaneous methylnaltrexone is effective
in inducing laxation in palliative care patients with
opioid-induced constipation and where conventional laxatives
have failed.
Key words: opioid-induced
constipation, methylnaltrexone, laxatives, cancer, management.
|
Background
Constipation is a common symptom in advanced cancer
patients. Studies have demonstrated that 40 to 80% of patients
on a palliative care service have constipation (Curtis, Krech,
Walsh, 1991; Sykes, 1998). This proportion increases to ?
90% when patients are treated with opioids (Sykes, 1998).
Fredericks, Hollis, & Carrie Stricker, (2010) define constipation
as less than three defecations per week (or change from usual
pattern), or the subjective symptom of difficult, infrequent,
or incomplete passage of stool that occurs in up to 90% of
patients with advanced cancer receiving opioids and can negatively
impact pain management and quality of life. Almost all patients
on opioids report constipation as the major side-effect. A
hospital survey showed that 87% of patients on strong opioids
required the use of laxatives. Among patients using morphine
80% reported constipation (Bouvy, Buurma, Egberts, 2002).
When opiates bind to the opiate receptors in the GI tract,
they interfere with peristalsis and the mucous secretion required
for bowel movements (Holzer, 2007; Mehendale, Yuan, 2006;
De, Cremonini, 2004; Holzer, 2004; Wood, Galligan, 2004; De,
Coupar, 1996). Use of exogenous opioids reduces peristalsis
(Mehendale et al., 2006), which, together with reduced secretion,
increased liquid reabsorption, and increased sphincter tone,
leads to the formation of dry, hard stools which are difficult
to pass (Pancha, Muller-Schwefe, Wurzelmann, 2007).
The impact of constipation on patients' quality of life is
important, especially for cancer patients (Choi & Billings,
2002) whose quality of life is already significantly impaired
by the illness itself. Constipation has been deemed by cancer
patients to be an even greater source of discomfort than the
pain they suffered (Fallon, 1999). According to World Health
Organization (WHO), opioids are very effective analgesics,
frequently prescribed in cancer pain (WHO, 1996). Despite
proven analgesic efficacy, the use of opioids is commonly
associated with frequently dose-limiting constipation that
seriously impacts on patients' quality of life (Reimer et
al., 2009). In addition to its negative impact on quality
of life, persistent constipation may lead to serious medical
sequelae, including bowel obstruction and fecal impaction,
may result in elevated use of prescription drugs and medical
services and may affect compliance with pain medications,
further compromising pain management strategies (Candrilli,
Davis, Iyer, 2009).
Therefore the purpose of this evidence-based review is to
answer the following PICOT question for an intervention/therapy,
where (P) stand for the population and primary problem, (I)
stand for intervention, (C) stand for comparison, (O) stand
for outcome, and (T) stand for time it takes to achieve an
outcome:
In patients with OIC, and they are cared for within the palliative
care unit (P), what is the effect of methylnaltrexone (I)
on the management of OIC (O) compared with laxatives (C) within
24 hours (T)?
Methods
Articles were retrieved for review via a combination of computer
and manual searches of selected opioid-induced constipation
and cancer-related publications. A comprehensive, and extensive
online database search of Science Direct Database, PubMed,
Springer Online Database, and HINARI/WHO Database was conducted
for opioid-induced constipation. Keywords used were "opioid-induced
constipation" "methylnaltrexone" "laxatives"
"cancer" "management" in multiple combination.
Also reference lists of related studies were searched.
The review utilized 6 articles, despite extensive search,
which met the inclusion criteria. The inclusion criteria were:
1. Randomized clinical trials (RCTs) 2. It investigated opioid-induced
constipation 3. Studies concerned adult participants receiving
palliative care. Based on this inclusion criteria a total
of 6 articles from 1991 to 2009 were selected and formed the
basis for this review.
Level of evidence of the included studies was rated based
on the work of Melnyk, Fineout-Overholt, (2005) and Stetler
et al., (1998). See table two in the appendix.
The six RCTs analyzed 498 participants; one study was of cross-over
design; the others were parallel design, of which three were
multi-center. The studies were undertaken in North American,
British, Spanish and Indian populations. All participants
were at an advanced stage of disease and were cared for within
a palliative care setting; most participants had a cancer
diagnosis. The average age of participants ranged from 61
to 72 years.
The drugs assessed were subcutaneous methylnaltrexone (Portenoy,
2008; Slatkin, 2009; Thomas, 2008) and the laxatives, all
taken orally, were senna (Agra, 1998; Ramesh, 1998; Sykes,
1991); lactulose (Agra, 1998; Sykes, 1991); danthron combined
with poloxamer (Sykes, 1991). One study also evaluated the
effect of misrakasneham; a drug used in traditional Indian
medicine as a purgative, containing castor oil, ghee, milk
and 21 kinds of herbs (Ramesh, 1998). In the studies on methylnaltrexone
nearly all participants (88% to 99%) were constipated at entry
despite taking one or more conventional laxatives.
Findings
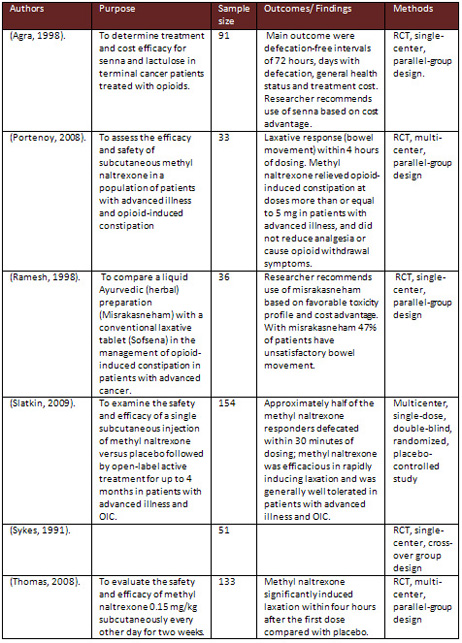
Descriptions of included studies in the review are displayed
through the table in the appendix.
Co-danthramer versus Senna plus Lactulose
One cross-over study of 51 participants evaluated the effectiveness
of co-danthramer versus senna plus lactulose (Sykes, 1991).
Both laxatives were in a liquid format.
Laxation responses: the researcher reports that
participants receiving 80 mg or more of strong opioid had
a significantly higher stool frequency when taking lactulose
plus senna than while receiving co-danthramer. While in a
lower dose of opioid, no statistical difference was reported.
Constipation-associated symptoms, pain intensity, opioid
withdrawal: not evaluated.
Acceptability and tolerability: diarrhea resulted
in suspension of laxative therapy for 24 hours for 15 patients
taking lactulose and for five patients taking codanthramer.
Researcher reported that six instances of diarrhea occurred
at opioid doses of at least 80 mg/day while taking lactulose
and senna; none were associated with co-danthramer. Two participants
reported perianal soreness and burning while taking codanthramer.
Participant preference was similar between the trial arms
(15 for lactulose and senna and 14 for co-danthramer), but
they also report that twice as many participants disliked
the flavor of co-danthramer compared to senna and lactulose.
Misrakasneham versus Senna
One small study of 36 participants evaluated the effectiveness
over two weeks of up to 10 ml of misrakasneham versus senna
24 mg to 72 mg (both in liquid format) (Ramesh, 1998).
Laxation responses: there was no statistical
difference between the misrakasneham and the senna groups
in satisfactory bowel movements. Six participants required
rescue laxatives, of which five were in the senna group.
Constipation-associated symptoms, pain intensity, opioid
withdrawal: not evaluated
.
Acceptability and tolerability: nausea, vomiting
and colicky pain were reported by two participants taking
misrakasneham. None of the participants withdrew due to inefficiency.
Participant preference was split between the groups.
Senna versus Lactulose
One study of 75 participants evaluated the effectiveness over
four weeks of lactulose 10 mg to 40 mg versus senna 12 mg
to 48 mg (both laxatives were in liquid format). Doses were
increased according to clinical response (Agra, 1998).
Laxation response: there was no statistical
difference between the senna and the lactulose groups in laxation
response. Thirty-seven percent of participants completing
the study required combined lactulose and senna to relieve
constipation.
Constipation-associated symptoms, pain intensity, opioid
withdrawal: there was no statistical difference in
the general state of health between the trial arms.
Acceptability and tolerability: three per trial
group, reported diarrhea, vomiting and cramps. There was no
significant difference in the number of participants who dropped
out between the trial arms.
Methylnaltrexone versus Placebo
Two studies evaluated subcutaneous methylnaltrexone versus
a placebo (Slatkin, 2009; Thomas, 2008). In one study a single
dose (0.15mg/kg or 0.30mg/kg) of methylnaltrexone was administered
(Slatkin, 2009); in the other study methylnaltrexone (0.15
mg/kg) was administered every other day for two weeks (Thomas,
2008).
Laxation response: participants who had a laxation
response at four hours ranged from 48% to 62% in the methylnaltrexone
trial groups and 13% to 15% in the placebo groups. At 24 hours
it was 52% to 68% in the active trial arms and 8% to 27% in
the placebo groups. A significant difference in laxation response
favoring the treatment group was also found in the multi dose
study at days three, five, seven, nine, eleven, and thirteen
(Thomas, 2008). In the single-dose study the researcher states
that the study demonstrated no dose-response relationship
(between 0.15 mg and 0.3 mg per kilogram doses) in laxation
and no correlation between laxation response and baseline
opioid dose (Slatkin, 2009). Dose response was not assessed
in the other study but at day eight, if participants had fewer
than three rescue-free laxations, the initial volume of the
study drug was doubled (to 0.30 mg of methylnaltrexone per
kilogram) (Thomas, 2008).
Constipation-associated symptoms, pain intensity, opioid
withdrawal: in the multi dose study they assessed
pain and symptoms of opioid withdrawal using the Modified
Himmelsbach Withdrawal Scale, at three time points; they found
no significant difference between the trial arms (Thomas,
2008). In the single-dose administration of methylnaltrexone
study there was no overall change from the baseline pain scores
or in having symptoms of opioid withdrawal (Slatkin, 2009).
Acceptability and tolerability: in the single-dose
study the researcher reports that during the double- blind
and subsequent open-label phase 19 participants experienced
severe adverse events that were possibly related to methylnaltrexone,
with some experiencing more than one event. These were: 15
incidents of abdominal pain, three of increased sweating,
two of increased pain and one each of burning at the injection
site, vomiting, diarrhea, asthenia, increased blood pressure,
dehydration, muscular cramps, loss of consciousness, tremor,
delirium, hallucination, dyspnea and flushing. In the same
study serious adverse events did not occur during the trial
phase but were reported in three participants during the subsequent
open-label phase. One participant had flushing and another
delirium possibly related to methylnaltrexone; a third had
severe diarrhea and subsequent dehydration and cardiovascular
collapse considered to be related to the drug (Slatkin, 2009).
In the other study they report that severe adverse events
occurred in 8% of participants in the methylnaltrexone group
and 13% in the placebo group (Thomas, 2008). The 11 serious
adverse events in those who received methylnaltrexone were:
aneurysm ruptured, respiratory arrest, dyspnea exacerbated,
suicidal ideation, aggression, malignant neoplasm progression,
concomitant disease progression, myocardial ischemia, coronary
artery disease aggravated and congestive heart failure aggravated.
The investigators considered all serious adverse events as
either not related or unlikely to be related to the trial
drug.
Dose Ranging Trial of Methylnaltrexone
One small study of 33 participants compared the effectiveness
of 1 mg (n = 10), 5 mg (n = 7), 12.5 mg (n =10) and 20 mg
(n =6) of subcutaneous methylnaltrexone (Portenoy, 2008).
Laxation response: the study reports that the
median time to laxation was 1.26 hours for patients dosed
at 5 mg or greater and in the 1mg group it was greater than
48 hours.
Constipation-associated symptoms, pain intensity, opioid
withdrawal: the researcher reports that there was
no evidence of methylnaltrexone-induced opioid withdrawal,
also there was not any difference in patient satisfaction
scores between the dose groups.
Acceptability and tolerability: all participants
experienced at least one treatment-emergent adverse event.
There was no significant difference between the lower dose
group compared to the other doses in the proportion of participants
who had a treatment related adverse event or discontinued
because of an adverse event. The types of adverse events were
similar between the dose groups. The most common adverse event
was abdominal pain. Two participants discontinued the trial
because of an adverse event. One was an 84-year old man who
withdrew due to syncope (12.5 mg dose). The event was transient
and resolved without sequelae; the investigators assessed
that it was related to the medication. A 20-year old man was
withdrawn after receiving three doses due to abdominal cramping,
assessed as probably related to the study medication.
Table 1: Description of included studies
Summary
This review sought to
determine the effectiveness of the administration of laxatives
and the opioid antagonist methylnaltrexone for the management
of constipation in palliative care patients. Six studies were
identified. Studies either compared the effectiveness of two
different laxatives, compared methylnaltrexone with a placebo
or different doses of methylnaltrexone. The effectiveness
of methylnaltrexone was not compared with a laxative and none
of the studies compared a laxative with a placebo; all comparisons
were made between different laxatives.
The review found that laxative use in the management of constipation
in this patient group is based on limited research evidence;
specifically, there have been no RCTs on any laxative that
have evaluated laxation response rate, patient tolerability
and acceptability.
There have been a few RCTs on the comparative advantages of
different laxatives. The limited evidence from these studies
suggests that the laxatives evaluated, including the commonly
used laxatives lactulose and senna, were of similar effectiveness
in this patient group. There is some evidence on the effectiveness
of methylnaltrexone, indicating that in comparison to placebo
and in patients where conventional laxative therapy is sub-optimal,
methylnaltrexone improves laxation.
References
Agra, Y., Sacristan, A., Gonzalez, M., Ferrari, M., Portugues,
A., Calvo, M.J. (1998). Efficacy of senna versus lactulose
in terminal cancer patients treated with opioids. Journal
of Pain and Symptom Management, 15, 1-7.
Bouvy, M.L., Buurma, H., Egberts, T.C.G. (2002). Laxative
prescribing in relation to opioid use and the influence of
pharmacy-based intervention. Journal of Clinical Pharmacy
and Therapeutics, 27, 107-110.
Candrilli, S.D., Davis, K.L., Iyer, S. (2009). Impact of Constipation
on Opioid Use Patterns, Health Care Resource Utilization,
and Costs in Cancer Patients on Opioid Therapy. Journal of
Pain & Palliative Care Pharmacotherapy, 23, (3), 231-241.
Chamberlain, B.H., Cross, K., Winston, J.L. (2009). Methylnaltrexone
treatment of opioid induced constipation in patients with
advanced illness. Journal of Pain and Symptom Management,
38, 683-90.
Choi, Y.S., Billings, J.A. (2002). Opioid antagonists: a review
of their role in palliative care, focusing on use in opioid-related
constipation. J Pain Symptom Manage, 24, 71-90.
Curtis, E.B., Krech, R., Walsh, T.D. (1991). Common symptoms
in patients with advanced cancer. J Palliat Care, 7, 25-29.
De Luca, A., and Coupar, I.M. (1996). Insights into opioid
action in the intestinal tract. Pharmacology & therapeutics
69, 103-15.
De Schepper, H.U., Cremonini, F., Park, M.I., et al. (2004).
Opioids and the gut: pharmacology and current clinical experience.
Neurogastroenterol Motil 16, 383-94.
Fallon, M.T. (1999). Constipation in cancer patients: Prevalence,
pathogenesis, and cost-related issues. European Journal of
Pain, 3, 3-7.
Fredericks, A., Hollis, G., Stricker, C.T. (2010). Diagnosis
and management of opioid-induced bowel dysfunction in patients
with advanced cancer. Clinical Journal of Oncology Nursing,
14, (6), 701-704.
Holzer, P. (2007). Treatment of opioid-induced gut dysfunction.
Expert opinion on investigational drugs, 16, 181-94.
Holzer, P. (2004). Opioids and opioid receptors in the enteric
nervous system: from a problem in opioid analgesia to a possible
new prokinetic therapy in humans. Neuroscience letters 361,
192-5.
Melnyck, B.M. & Fineout-Overholt, E. (2005). Evidence-based
practice in healthcare. Philadelphia: Lippincott.
Mehendale, S.R, and Yuan, C.S (2006). Opioid-induced gastrointestinal
dysfunction. Dig Dis 24, 105-12.
Organization, WH (1996). Cancer pain relief. (Geneva: WHO).
Panchal, S.J., Muller-Schwefe, P., Wurzelmann, J.I. (2007).
Opioid-induced bowel dysfunction: prevalence, pathophysiology
and burden. Int J Clin Pract, 61, 1181-7.
Portenoy, R.K., Thomas, J., Moehl, Boatwright, M.L., Galasso,
F.L., Stambler, N., Von Gunten, C.F., et al. (2008). Subcutaneous
methylnaltrexone for the treatment of opioid-induced constipation
in patients with advanced illness: a double-blind, randomized,
parallel group, dose-ranging study. Journal of Pain and Symptom
Management, 35, 458-68.
Ramesh, P., Suresh Kumar, K., Rajagopal, M., Balachandran,
P., Warrier, P. (1998). Managing morphine-induced constipation:
a controlled comparison of the ayurvedic formulation and senna.
Journal of Pain and Symptom Management, 16, (4), 240-4.
Reimer, K., Hopp, M., Zenz, M., et al. (2009). Meeting the
challenges of opioid-induced constipation in chronic pain
management-a novel approach. Pharmacology, 83, 10-17.
Slatkin, N., Thomas. J., Lipman, A.G., Wilson, G., Boatwright,
M.L., Wellman, C., et al. (2009). Methylnaltrexone for treatment
of opioid-induced constipation in advanced illness patients.
Journal of Supportive Oncology, 7, 39-46.
Stetler, C.B., Morsi, D., Rucki, S., Broughton, S., Corrigan,
B., Fitzgerald, J., et al. (1998). Utilization-focused integrative
reviews in a nursing service. Applied Nursing Research, 11,
195-206.
Sykes, N. (1991). A clinical comparison of laxatives in a
hospice. Palliative Medicine, 5, 307-14.
Sykes, N.P. (1998). Constipation and Diarrhea. In: Doyle,
D., Hanks, G., MacDonald, N., eds. Oxford textbook of palliative
medicine. Oxford: Oxford University Press, 513-526.
Wood, J.D, and Galligan, J.J. (2004). Function of opioids
in the enteric nervous system. Neurogastroenterol Motil. 16,
17-28.
|
 |




